- Research
- Open access
- Published:
Efficacy and toxicity of KRASG12C inhibitors in advanced solid tumors: a meta-analysis
World Journal of Surgical Oncology volume 22, Article number: 182 (2024)
Abstract
Background
The efficacy and toxicity of KRASG12C inhibitors were evaluated for advanced solid tumors in several studies; however, the results were not fully consistent.
Methods
Clinical trials evaluating KRASG12C inhibitors for advanced solid tumors were searched from PubMed, Embase, and Cochrane Library online databases up to 31st December 2023. The characteristics of the studies and the results of objective response rate (ORR), disease control rate (DCR), duration of response (DoR), progression-free survival (PFS) rate, overall survival (OS) rate, and treatment-related adverse events (trAEs) were extracted.
Results
Ten studies with 925 heavily pretreated advanced patients harboring KRASG12C mutation were included. For total population, the pooled analysis of ORR was 28.6% (95%CI, 21.2-36.6%), DCR was 85.5% (95%CI, 82.2-88.6%), PFS rate at 6 months (PFS6) was 49.6% (95%CI, 41.4-57.9%), PFS rate at 12 months (PFS12) was 26.7% (95%CI, 19.8-34.1%), OS rates at 6 months (OS6) was 76.2% (95%CI, 68.8-82.9%), OS rates at 12 months (OS12) was 47.8% (95%CI, 38.6-57.0%). The pooled analysis of any grade trAEs was 79.3% (95%CI, 66.2-90.0%) and grade three or more trAEs was 24.4% (95%CI, 16.7-32.9%). The median time to response and DoR results from individual data were 1.39 months (95%CI, 1.37–1.41 months) and 10.54 months (95%CI, 7.72–13.36 months). Sotorasib had significantly lower pooled incidences of any trAEs (OR, 0.07, 95%CI, 0.03–0.14) and grade three or more trAES (OR, 0.34, 95%CI, 0.24–0.49) compared with adagrasib.
Conclusions
KRASG12C inhibitors have good ORR, DCR, PFS rate, OS rate, tolerable trAEs, and early response with long duration in advanced solid tumors; however, most of the pooled results were heterogeneous. Sotorasib has shown better safety results.
Background
Kirsten rat sarcoma (KRAS) gene is one of the most frequently mutated oncogenes, and it regulates cell signaling through downstream mitogen activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways by encoding its protein [1]. KRAS mutations can cause active state of tyrosine kinase and constant activation of downstream signaling which lead to cell proliferation, migration, and tumorigenesis [2,3,4]. Despite KRAS mutations may play an important role in tumorigenesis, there has been no meaningful progress in targeted therapy against these mutations [5]. KRASG12C mutation (glycine-to-cysteine substitution at codon 12) is one of the most common KRAS mutations, and it may occur in a variety of tumor types, such as non-small-cell lung cancer (NSCLC), colorectal cancers (CRC), pancreatic cancers, and other cancers [6].
In recent years, a new binding site on the KRASG12C protein was found, and several small molecule drugs targeting the KRASG12C mutation have been developed [7,8,9]. Sotorasib, the first KRASG12C inhibitor, can irreversibly and specifically inhibit the KRASG12C protein, prevent downstream signaling, and thereby hinder oncogenesis [10]. In 2020, a phase 1 trial of sotorasib in patients with advanced solid tumors with KRASG12C mutation suggested promising anticancer activity and tolerable toxicity in heavily pretreated patients [11]. Subsequent phase 2 and 3 clinical trials for NSCLC, CRC, and pancreatic cancers also indicated encouraging results [12,13,14,15]. Other KRASG12C inhibitors such as adagrasib, garsorasib, and divarasib were also investigated in several clinical trials [16,17,18,19,20]. The phase 1 KRYSTAL-1 study suggested that adagrasib was well tolerated and effective for advanced solid tumor patients with KRASG12C mutation. Another phase 1 study evaluating divarasib for advanced solid tumor patients with KRASG12C mutation showed durable clinical responses with mostly low-grade adverse events. A more recent phase 1 study evaluating garsorasib for NSCLC patients with KRASG12C mutation also presented encouraging antitumor activity and good safety profiles. However, the results of KRASG12C inhibitors were not fully consistent.
We searched clinical trials that evaluating KRASG12C inhibitors for advanced solid tumors from online databases. And we conducted a meta-analysis to evaluate the efficacy and toxicity of KRASG12C inhibitors in advanced solid tumors with KRASG12C mutation.
Methods
Data sources and search strategy
Studies were searched via PubMed, Embase, and Cochrane Library database up to 31st December 2023. We used the Medical Subject Headings (MeSH) terms and their entry terms as follows: “KRAS G12C OR sotorasib OR lumakras OR AMG-510 OR AMG 510 OR AMG510 OR adagrasib OR MRTX849 OR MRTX-849 OR ARS853 OR ARS-1620 OR garsorasib OR BI-2852”.
Inclusion and exclusion criteria
This meta-analysis followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement [21].
The inclusion criteria were as follows: (a) patients with KRASG12C mutation advanced solid tumors; (b) there was at least one group that was treated with KRASG12C inhibitors monotherapy; (c) there was at least one outcome of objective response rate (ORR), disease control rate (DCR), duration of response (DoR), progression-free survival (PFS), overall survival (OS), treatment-related adverse events (trAEs) was reported.
The exclusion criteria were as follows: (a) patients were treated with KRASG12C inhibitors plus other systemic therapy; (b) the data could not be extracted; (c) the study was not published in English; (d) retrospective study, conference abstracts, case reports, comments, reviews, animal studies, and mechanistic studies.
Quality assessment and data extraction
The risk of bias was assessed by two investigators (Shoutao Dang and Shuyang Zhang) according to the Methodological Index for Nonrandomized Studies (MINORS) tool [22].
The data were extracted by the same two investigators. The following information were included: first author, publication year, trial phase, sample size, eligible patients, interventions and control group, and outcomes of the ORR, DCR, DoR, PFS, OS, and trAEs. The PFS rates, OS rates, and individual DoR data were indirectly extracted from survival curves and swimmer plot figures. Any disagreement was discussed for consensus.
Statistical analysis
This meta-analysis was performed by Stata 17. The pooled results of ORR, DCR, PFS rate, OS rate, and incidences of trAEs were performed by “metaprop” statistical program. The subgroup pooled results were performed by “subgroup by” statistical program. The heterogeneity of the studies was estimated by using the I2 statistics. Random-effects model was used for all results. Kaplan-Meier estimates for extracted individual data of DoR were produced by “graph Kaplan-Meier survivor function”. Log-rank test was calculated by “sts test” statistical program to compare among different subgroups of DoR. The odds ratio (OR) was calculated by “csi” statistical program to compare the pooled incidences between different subgroups. Statistical significance was set at p < 0.05.
Results
Study and data selection
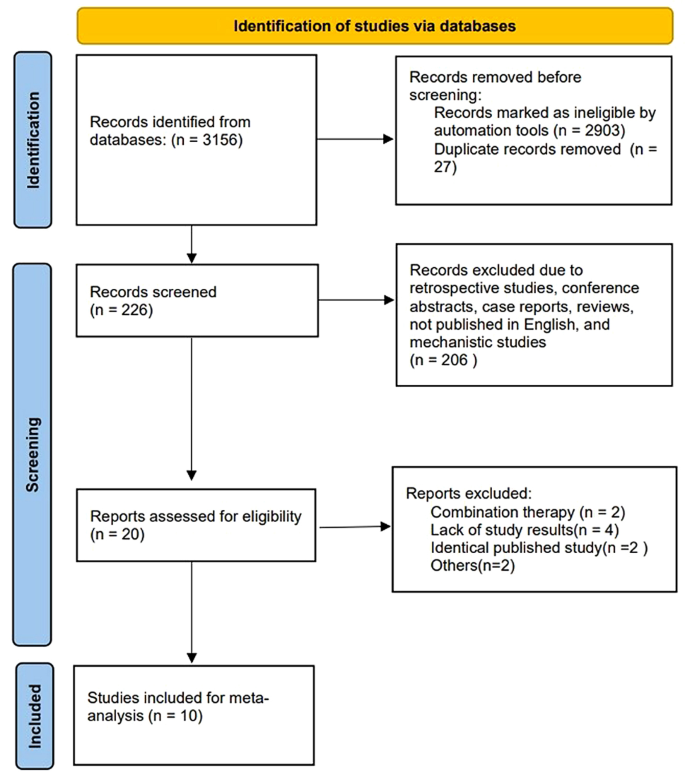
The study selection process is summarized in Fig. 1. We retrieved 3156 records from the primary search strategy, of which 2903 were removed for non-clinical trials. Twenty-seven records were excluded for duplicate results. Two hundred and six records were further excluded due to retrospective studies, conference abstracts, case reports, reviews, not published in English, and mechanistic studies. Twenty studies were assessed for eligibility, and ten studies were excluded (combination therapy = 2, lack of study results = 4, identical published study = 2, others = 2). Ultimately, ten studies with 925 patients were enrolled in this meta-analysis.
Study characteristics and quality assessment
The characteristics of the ten studies are shown in Table 1. Five studies used sotorasib [11,12,13,14,15], three studies used adagrasib [16, 18, 20], the other two studies used divarasib [19] and garsorasib [17], respectively. Seven studies reported NSCLC patients’ results [11, 12, 15,16,17,18,19], five studies reported CRC patients’ results [11, 14, 18,19,20], and four studies reported other (mostly pancreatic cancer and a variety of other tumors such as appendix, bile duct, endometrial, gastric and so on) patients’ results [11, 13, 18, 19]. The vast majority of studies included patients with a median No. of previous treatment lines ≥ 2. For NSCLC population, five studies were median No. of previous treatment lines = 2, the other one was 3. However, for CRC population, all four studies were median No. of previous treatment lines ≥ 3. And for other solid tumors, one study was median No. of previous treatment lines = 1, one was 2, and the other one was 3. Nine studies reported the results of median follow-up, and the shortest median follow-up time was 8.8 months (95% CI, 0.7–14.9).
The nine non-randomized controlled trials (RCT) studies without the control group scored 12–14 of 16, and the other RCT study scored 24 of 24 on MINORS (Supplement Table S1). The MINORS results suggested consistent high methodological quality for the included studies.
Pooled analysis of ORR and DCR
The pooled results of ORR were 28.6% (95%CI, 21.2-36.6%, I2 = 81.23%) for total population, 40.0% (95%CI, 32.2-48.2%, I2 = 73.21%) for NSCLC patients, 16.3% (95%CI, 5.7-30.1%, I2 = 76.78%) for CRC patients, 19.5% (95%CI, 9.5-31.6%, I2 = 24.35%) for other cancers (Fig. 2A), 23.0% (95%CI, 15.0-32.2%, I2 = 80.43%) for sotorasib monotherapy, 33.2% (95%CI, 17.9-50.5%, I2 = 76.65%) for adagrasib monotherapy (Fig. 2B), 36.3% (95%CI, 26.9-46.2%, I2 = 78.15%) for median NO. of previous treatment lines ≤ 2, and 26.7% (95%CI, 13.6-41.9%, I2 = 75.14%) for median NO. of previous treatment lines > 2 (Supplement Figure S1A). NSCLC had significantly higher pooled ORR compared with CRC (OR, 3.36, 95%CI, 2.56–5.02, p < 0.0001) and other cancers (OR, 2.70, 95%CI, 1.58–4.60, p = 0.0002). Sotorasib had significantly lower pooled ORR compared with adagrasib (OR, 0.60, 95%CI, 0.42–0.87, p = 0.0073). The patients with median NO. of previous treatment lines ≤ 2 had significantly higher pooled ORR compared with > 2 lines (OR, 1.56, 95%CI, 1.09–2.22, p = 0.0137, Table 2).
The pooled results of DCR were 85.5% (95%CI, 82.2-88.6%, I2 = 29.81%) for total population, 86.3% (95%CI, 81.3-90.8%, I2 = 60.67%) for NSCLC patients, 84.1% (95%CI, 78.3-89.3%, I2 = 0.00%) for CRC patients, 83.5% (95%CI, 74.0-91.5%, I2 = 0.00%) for other cancers (Fig. 2C), 81.5% (95%CI, 78.0-84.7%, I2 = 0.00%) for sotorasib monotherapy, 85.8% (95%CI, 76.3-93.3%, I2 = 51.56%) for adagrasib monotherapy (Fig. 2D), 85.4% (95%CI, 80.9-89.4%, I2 = 37.64%) for median NO. of previous treatment lines ≤ 2, and 88.5% (95%CI, 82.5-93.6%, I2 = 14.52%) for median NO. of previous treatment lines > 2 (Supplement Figure S1B). The pooled DCR was not significantly different between NSCLC and CRC (OR, 1.21, 95%CI, 0.78–1.87, p = 0.397), NSCLC and others (OR, 1.25, 95%CI, 0.69–2.26, p = 0.465), sotorasib and adagrasib (OR, 0.72, 95%CI, 0.45–1.16, p = 0.18), median NO. of previous treatment lines ≤ 2 and > 2 (OR, 0.75, 95%CI, 0.46–1.22, p = 0.25, Table 2).
Pooled analysis of time to response and DOR
There were 221 individual data of time to response and DoR were extracted. The median time to response was 1.39 months (95%CI, 1.37–1.41 months) for total population, 1.41 months (95%CI, 1.38–1.44 months) for sotorasib monotherapy, 1.36 months (95%CI, 1.33–1.39 months) for adagrasib monotherapy, and 1.38 months (95%CI, 1.33–1.43 months) for garsorasib monotherapy.
The median DoR was 10.54 months (95%CI, 7.72–13.36 months) for total population, 11.06 months (95%CI, 8.02–14.10 months) for NSCLC patients, 5.68 months (95%CI, 4.13–7.23 months) for non-NSCLC patients (Fig. 3A), 10.77 months (95%CI, 7.42–14.12 months) for sotorasib, 8.49 months (95%CI, 3.63–13.35 months) for adagrasib, not evaluable for garsorasib (Fig. 3B). NSCLC patients had significantly better DoR than non-NSCLC patients (Log-rank, p = 0.009). However, DoR was not significantly different among the three drugs (Log-rank, sotorasib VS adagrasib, p = 0.35; sotorasib VS garsorasib, p = 0.92; adagrasib VS garsorasib, p = 0.41).
Pooled analysis of PFS
The pooled PFS rates at 6 months (PFS6) were 48.2% (95%CI, 40.2-56.3%, I2 = 80.73%) for total population, 56.7% (95%CI, 49.3-64.0%, I2 = 67.32%) for NSCLC patients, 35.7% (95%CI, 23.4-49.0%, I2 = 72.73%) for CRC patients (Fig. 4A), 39.4% (95%CI, 29.6-49.6%, I2 = 79.22%) for sotorasib monotherapy, 51.5% (95%CI, 43.8-59.1%, I2 = 0.00%) for adagrasib monotherapy (Supplement Figure S2A), 54.1% (95%CI, 44.5-63.6%, I2 = 80.30%) for median NO. of previous treatment lines ≤ 2, and 42.6% (95%CI, 26.6-59.3%, I2 = 78.02%) for median NO. of previous treatment lines > 2 (Supplement Figure S2B).The pooled PFS rates at 12 months (PFS12) were 26.7% (95%CI, 19.8-34.1%, I2 = 78.79%) for total population, 32.3% (95%CI, 24.9-40.2%, I2 = 73.20%) for NSCLC patients, 13.7% (95%CI, 8.6-19.6%, I2 = 0.00%) for CRC patients (Fig. 4B), 22.0% (95%CI, 16.0-28.7%, I2 = 56.90%) for sotorasib monotherapy, 28.0% (95%CI, 13.2-45.5%, I2 = 75.54%) for adagrasib monotherapy (Supplement Figure S2C), 32.5% (95%CI, 24.1-41.6%, I2 = 78.43%) for median NO. of previous treatment lines ≤ 2, and 18.8% (95%CI, 8.7-31.3%, I2 = 70.20%) for median NO. of previous treatment lines > 2 (Supplement Figure S2D).
NSCLC had significantly higher pooled PFS6 (OR, 2.36, 95%CI, 1.70–3.28, p < 0.0001) and PFS12 (OR, 2.97, 95%CI, 1.93–4.56, p < 0.0001) compared with CRC. Sotorasib had significantly lower pooled PFS6 compared with adagrasib (OR, 0.61, 95%CI, 0.43–0.87, p = 0.0057); however, the pooled PFS12 was not significantly different (OR, 0.73, 95%CI, 0.49–1.08, p = 0.11). The patients with median NO. of previous treatment lines ≤ 2 had significantly higher pooled PFS6 (OR, 1.58, 95%CI, 1.13–2.23, p = 0.0078) and PFS12 (OR, 2.09, 95%CI, 1.38–3.17, p = 0.0005) compared with > 2 lines (Table 2).
Pooled analysis of OS
The pooled OS rates at 6 months (OS6) were 76.2% (95%CI, 68.8-82.9%, I2 = 70.55%) for total population, 73.0% (95%CI, 68.6-77.2%, I2 = 0.00%) for NSCLC patients, 88.1% (95%CI, 81.1-93.8%, I2 = not applicable) for CRC patients (Fig. 4C), 73.6% (95%CI, 65.4-81.1%, I2 = 64.17%) for sotorasib monotherapy, 80.9% (95%CI, 62.0-94.7%, I2 = 82.01%) for adagrasib monotherapy (Supplement Figure S3A), 71.2% (95%CI, 66.0-76.2%, I2 = 28.19%) for median NO. of previous treatment lines ≤ 2, and 86.4% (95%CI, 75.9-94.5%, I2 = 47.16%) for median NO. of previous treatment lines > 2 (Supplement Figure S3B). The pooled OS rates at 12 months (OS12) were 47.8% (95%CI, 38.6-57.0%, I2 = 76.58%) for total population, 49.6% (95%CI, 44.8-54.5%, I2 = 0.00%) for NSCLC patients, 53.0% (95%CI, 43.3-62.5%, I2 = not applicable) for CRC patients (Fig. 4D), 40.7% (95%CI, 29.5-52.4%, I2 = 79.55%) for sotorasib monotherapy, 58.9% (95%CI, 46.4-70.9%, I2 = 51.23%) for adagrasib monotherapy (Supplement Figure S3C), 43.1% (95%CI, 32.3-54.3%, I2 = 80.94%) for median NO. of previous treatment lines ≤ 2, and 56.7% (95%CI, 37.9-74.7%, I2 = 73.59%) for median NO. of previous treatment lines > 2 (Supplement Figure S3D).
NSCLC had significantly lower pooled OS6 (OR, 0.38, 95%CI, 0.20–0.69, p = 0.0015) compared with CRC; however, the pooled OS12 was not significantly different (OR, 0.88, 95%CI, 0.58–1.35, p = 0.56). Sotorasib had significantly lower pooled OS12 compared with adagrasib (OR, 0.48, 95%CI, 0.33–0.68, p = 0.0001); however, the pooled OS6 was not significantly different (OR, 0.67, 95%CI, 0.43–1.03, p = 0.066). The patients with median NO. of previous treatment lines ≤ 2 had significantly lower pooled OS6 (OR, 0.40, 95%CI, 0.23–0.69, p = 0.0008) and OS12 (OR, 0.58, 95%CI, 0.39–0.87, p = 0.0077) compared with > 2 lines (Table 2).
Pooled analysis of trAEs
The pooled incidences of any grade trAEs were 79.3% (95%CI, 66.2-90.0%, I2 = 94.89%) for total population, 60.3% (95%CI, 51.2-69.0%, I2 = 75.72%) for sotorasib monotherapy, 95.9% (95%CI, 91.3-99.0%, I2 = 22.82%) for adagrasib monotherapy (Fig. 5A). The pooled incidences of grade three or more trAEs were 24.4% (95%CI, 16.7-32.9%, I2 = 87.42%) for total population, 18.5% (95%CI, 10.8-27.6%, I2 = 82.72%) for sotorasib monotherapy, 39.9% (95%CI, 32.8-47.2%, I2 = 0.00%) for adagrasib monotherapy (Fig. 5B). Sotorasib had significantly lower pooled incidences of any trAES (OR, 0.07, 95%CI, 0.03–0.14, p < 0.0001) and grade three or more trAEs (OR, 0.34, 95%CI, 0.24–0.49, p < 0.0001) compared with adagrasib (Table 2).
There were 20 specific trAEs which were reported in at least three studies (Supplement Table S2). Diarrhoea, nausea, vomiting, fatigue, ECG QT prolonged, aspartate aminotransferase (AST) increased, alanine aminotransferase (ALT) increased, amylase increase, blood creatinine increase/acute kidney injury, decreased appetite, and anaemia were the very common (≥ 10%) any grade trAEs. Alkaline phosphatase increased, lipase increased, dysgeusia, edema peripheral, hyponatremia, abdominal pain, pneumonitis, lymphocyte count decrease, and neutrophil count decrease were the common (< 10%, ≥ 1%) any grade trAEs.
ALT increased, AST increased, diarrhoea, ECG QT prolonged, lipase increased, anaemia, fatigue, alkaline phosphatase increased, amylase increased, and hyponatremia were the common grade three or more trAEs. Blood creatinine increase/acute kidney injury, pneumonitis, lymphocyte count decrease, decreased appetite, nausea, neutrophil count decrease, abdominal pain, and vomiting were the uncommon (< 1%, ≥ 0.1%) grade three or more trAEs (Supplement Table S3). The other reported grade three or more specific trAEs were rare (< 0.1%, ≥ 0.01%) or very rare (< 0.01%).
Subgroup analyses for the incidences of the 20 specific trAEs by different drugs are also shown in Table S2 and Table S3. Most of the any grade specific trAEs were more common for adagrasib compared with sotorasib. However, for the grade three or more specific trAEs, some were more common for adagrasib while some others were not.
Publication bias analysis
The publication bias was analyzed by Egger’s test. The p-values of ORR, DCR, PFS6, PFS12, OS6, OS12, incidence of any trAEs, and incidence of grade three or more trAEs were 0.454, 0.402, 0.740, 0.671, 0.676, 0.996, 0.974, and 0.846, respectively (Supplement Figure S4). These results did not indicate any publication bias.
Discussion
KRAS mutations are common in a variety of cancers, and they are considered to have some impact on the prognoses of cancer patients [23,24,25]. Specific KRASG12C mutation inhibitors such as sotorasib, adagrasib, divarasib, and garsorasib have been evaluated in recent years and have shown encouraging results. A recent meta-analysis reported the pooled results for KRASG12C inhibitors in solid tumors. However, that study included combination therapy as well as monotherapy and only ORR, DCR, and ≥ grade three AEs were reported for KRASG12C inhibitors monotherapy [26]. Our study suggested that KRASG12C inhibitors monotherapy had good ORR, DCR, PFS rate, OS rate, tolerable trAEs, and early response with long duration in advanced solid tumors.
For total population, the pooled analysis of ORR was 28.6% (95%CI, 21.2-36.6%), DCR was 85.5% (95%CI, 82.2-88.6%), PFS rate at 6 months (PFS6) was 49.6% (95%CI, 41.4-57.9%), PFS rate at 12 months (PFS12) was 26.7% (95%CI, 19.8-34.1%), OS rates at 6 months (OS6) was 76.2% (95%CI, 68.8-82.9%), OS rates at 12 months (OS12) was 47.8% (95%CI, 38.6-57.0%). The pooled analysis of any grade trAEs was 79.3% (95%CI, 66.2-90.0%), and grade three or more trAEs was 24.4% (95%CI, 16.7-32.9%). The median time to response and DoR results from individual data were 1.39 months (95%CI, 1.37–1.41 months) and 10.54 months (95%CI, 7.72–13.36 months). It should be noted that most of the studies included in the meta-analysis enrolled previously heavily treated patients who were prone to resist systemic therapy and lack of treatment options. Therefore, the pooled results of our study indicated that KRASG12C inhibitors had good ORR, DCR, PFS rate, OS rate, tolerable trAEs, and early response with long duration. These results supported the use of KRASG12C inhibitors in advanced solid tumors.
For subgroup analysis by cancer types, our study suggested that NSCLC had significantly better pooled ORR, DoR, and PFS rate compared with CRC and/or others. However, the pooled OS6 was significantly lower compared with CRC. The pooled results for NSCLC were supported by a recent phase 3 RCT study that advanced NSCLC with KRASG12C mutation who progressed after previous platinum-based chemotherapy and immune checkpoint inhibitors were randomly assigned (1:1) to oral sotorasib or intravenous docetaxel [15]. In this study, the ORR, DCR, and median DoR were 28.1%, 82.5%, and 8.6 months for sotorasib group, and 13.2%, 60.3%, and 6.8 months for docetaxel group. The primary endpoint PFS was statistically significantly increased by sotorasib with fewer grade three or more trAEs compared with docetaxel. However, the median PFS improvement was modest clinically meaningful (from 4.5 months to 5.6 months), and the OS was not significantly different (HR, 1.01, 95%CI, 0.77–1.33). These results led to questionable efficacy of sotorasib for NSCLC with KRASG12C mutation [27]. Despite negative OS results in this phase III trial, sotorasib still had higher ORR and DCR, longer DoR, and fewer trAEs compared with docetaxel. It should also be noted that this study was not powered for OS and there were 33.9% patients in the chemotherapy group crossed over to sotorasib group. Further studies are needed to select suitable patients for KRASG12C inhibitors. Another ongoing phase 3 RCT study (NCT04685135) evaluating adagrasib versus docetaxel in advanced NSCLC with KRASG12C mutation may provide more evidences for whether KRASG12C inhibitors are superior to standard care therapy (SOC). Despite the encouraging results for KRASG12C inhibitors monotherapy in NSCLC, the results in CRC and other cancers were less satisfactory. KRASG12C inhibitors combined therapy was investigated in another phase 3 RCT study that chemorefractory metastatic CRC patients with KRASG12C mutation were randomly assigned in a 1:1:1 ratio to receive sotorasib (960 mg once daily) plus panitumumab, sotorasib (240 mg once daily) plus panitumumab, and the investigator’s choice of SOC [28]. The ORR, DCR, and median DoR were 26.4%, 71.7%, and 4.4 months for sotorasib (960 mg) combination group, 5.7%, 67.9%, and not estimated for sotorasib (240 mg) combination group, 0%, 46.3%, and not estimated for SOC group. The primary endpoint PFS was also significantly increased by sotorasib (960 mg) combination (HR, 0.49, 95%CI, 0.30 to 0.80; P = 0.006) and sotorasib (240 mg) combination (HR, 0.58,95%CI, 0.36 to 0.93; P = 0.03) compared with SOC group. KRASG12C inhibitor plus an EGFR inhibitor had better efficacy than SOC in CRC; however, this combination therapy seemed to have similar or even worse ORR, DCR, and DoR compared with our pooled results. EGFR inhibitor was considered to be ineffective for RAS mutated CRC [29, 30]. Whether this combination therapy is superior compared with KRASG12C inhibitor monotherapy is still unclear. Further studies are required to investigate the reasonable combination therapy strategy for KRASG12C mutated solid tumors beyond NSCLC. Regarding other tumors, pancreatic cancer is another common solid tumor with KRASG12C mutation. A phase 1–2 study assessed sotorasib in pancreatic cancer who had received at least one previous systemic therapy and showed anticancer activity. The other three phase 1 studies assessed sotorasib, adagrasib, and divarasib in solid tumors other than NSCLC and CRC. However, due to fewer patients included and early phase of the trials, the PFS and OS results could not be pooled. The pooled ORR seemed to be similar with CRC. KRASG12C inhibitor might be an option for these patients.
For subgroup analysis by drugs, our study suggested that sotorasib had significantly lower pooled ORR, PFS6, and OS12 compared with adagrasib; however, the DCR, DoR, PFS12, and OS6 were not significantly different. Sotorasib also had significantly lower pooled incidences of any trAEs and grade three or more trAEs. And most of the any grade specific trAEs were more common for adagrasib compared with sotorasib. The mean half-life was 5.5 h for sotorasib and 23 h for adagrasib [11, 18], and sotorasib was applied once daily while adagrasib was applied twice daily. Therefore, we speculated that adagrasib might have more accumulated toxicity than sotorasib due to its longer half-life and more frequency of administration. The accumulated dose of adagrasib might also be one of the reasons for higher pooled ORR compared with sotorasib because high dose of KRASG12C inhibitor seemed to have better ORR than low dose in a CRC phase 3 RCT study [28]. However, it should be noted that adagrasib included a relatively higher proportion of NSCLC (72.1% VS 67.7%) whose ORR is better than non-NSCLC and only phase 1–2 study with fewer patients (183 VS 526) which might be more heterogeneous. Therefore, the efficacy conclusions should be made carefully between the two drugs. Divarasib and garsorasib were also assessed in phase 1 study. Both drugs seemed to have similar ORR and DCR compared with adagrasib. Regarding safety profiles, divarasib seemed to have a higher incidence of any trAEs but similar grade three or more trAEs compared with sotorasib. And garsorasib seemed to have similar incidences of any trAEs and grade three or more trAEs compared with adagrasib. However, no conclusion could be indicated because there was only one phase 1 study for each drug. Further studies are needed to evaluate the efficacy and toxicity results among different KRASG12C inhibitors.
For subgroup analysis by previous treatment lines, the patients with median NO. of previous lines ≤ 2 had significantly higher pooled ORR, PFS6, and PFS12 compared with > 2 lines; however, the pooled OS6 and OS12 were significantly lower for ≤ 2 lines. We should note that most patients with median NO. of previous lines ≤ 2 were NSCLC and most patients with > 2 lines were CRC. Therefore, it is hard to say whether these differences were caused by different tumors or previous treatment lines.
Most of our pooled results were heterogeneous even for subgroup analysis by different cancers and drugs. The heterogeneity might be mainly due to the non-RCT studies with different baseline characteristics of the patients such as race, number of lines of previous anticancer therapy, previous drugs used, and co-mutation status. KEAP1 co-mutations were prone to have poorer outcomes for patients treated with KRASG12C inhibitors and were considered to be associated with resistance [1, 26]. RTK-RAS pathway and switch-II binding pocket mutations might be the mechanisms of acquired resistance to KRASG12C inhibitors [31]. KRASG12C inhibitors combination therapy and pan-RAS inhibitors may help to improve efficacy for patients with KRASG12C mutation.
We conducted a comprehensive analysis of the efficacy and toxicity characteristics of KRASG12C inhibitors monotherapy in advanced solid tumors. Our pooled results contained ORR, DCR, DoR, PFS rate, OS rate, and incidences of any trAEs, grade three or more trAEs, and specific trAEs. These results may help us to understand more about the characteristics of this treatment. However, our study had limitations. Firstly, most of our pooled results were heterogeneous, and the sources of heterogeneity were still unclear. Additionally, the comparison among different subgroups was not head-to-head, and further studies are required to confirm these results. Furthermore, most of the included trials were phase 1 or 2 non-RCT studies which might cause biases and heterogeneities.
Conclusions
KRASG12C inhibitors had good ORR, DCR, PFS rate, OS rate, tolerable trAEs, and early response with long duration in advanced solid tumors; however, most of the pooled results were heterogeneous. NSCLC had significantly better pooled ORR, DoR, and PFS rate compared with CRC and/or others. However, the pooled OS6 was significantly lower compared with CRC. Sotorasib had significantly lower pooled ORR, PFS6, and OS12 compared with adagrasib; however, the DCR, DoR, PFS12, and OS6 were not significantly different. Sotorasib also had significantly lower pooled incidences of any trAEs and grade three or more trAEs compared with adagrasib. The patients with median NO. of previous lines ≤ 2 had significantly higher pooled ORR, PFS6, and PFS12 compared with > 2 lines; however, the pooled OS6 and OS12 were significantly lower for ≤ 2 lines.
Data availability
The data underlying this article are available in the article and in its supplementary material.
Abbreviations
- KRAS:
-
Kirsten rat sarcoma.
- MAPK:
-
Mitogen activated protein kinase
- PI3K:
-
Phosphatidylinositol 3-kinase
- NSCLC:
-
Non-small-cell lung cancer
- CRC:
-
Colorectal cancers
- MeSH:
-
Medical Subject Headings
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- ORR:
-
Objective response rate
- DCR:
-
Disease control rate
- DoR:
-
Duration of response
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- trAEs:
-
Treatment related adverse events
- MINORS:
-
Methodological Index for Nonrandomized Studies
- OR:
-
Odds ratio
- RCT:
-
Randomized controlled trial
- PFS6:
-
PFS rates at 6 months
- PFS12:
-
PFS rates at 12 months
- PFS6:
-
OS rates at 6 months
- PFS12:
-
OS rates at 12 months
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
References
O’Leary C, Murphy G, Yeung Y, Tang M, Jain V, O’Leary CG. Targeted therapies for Kirsten Rat Sarcoma (KRAS) G12C mutant metastatic non-small-cell lung cancers. Cancers (Basel). 2023;15(23).
Murugan AK, Grieco M, Tsuchida N. RAS mutations in human cancers: roles in precision medicine. Semin Cancer Biol. 2019;59:23–35.
Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410(6832):1111–6.
Wu X, Song W, Cheng C, Liu Z, Li X, Cui Y, et al. Small molecular inhibitors for KRAS-mutant cancers. Front Immunol. 2023;14:1223433.
Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: Mission possible? Nat Rev Drug Discov. 2014;13(11):828–51.
Yang Y, Zhang H, Huang S, Chu Q. KRAS mutations in solid tumors: characteristics, current therapeutic strategy, and potential treatment exploration. J Clin Med. 2023;12(2).
Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217–23.
Janes MR, Zhang J, Li LS, Hansen R, Peters U, Guo X, et al. Targeting KRAS Mutant Cancers with a covalent G12C-Specific inhibitor. Cell. 2018;172(3):578–e8917.
Nagasaka M, Li Y, Sukari A, Ou SI, Al-Hallak MN, Azmi AS. KRAS G12C Game of thrones, which direct KRAS inhibitor will claim the iron throne? Cancer Treat Rev. 2020;84:101974.
Lanman BA, Allen JR, Allen JG, Amegadzie AK, Ashton KS, Booker SK, et al. Discovery of a covalent inhibitor of KRAS(G12C) (AMG 510) for the treatment of solid tumors. J Med Chem. 2020;63(1):52–65.
Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRAS(G12C) inhibition with Sotorasib in Advanced Solid tumors. N Engl J Med. 2020;383(13):1207–17.
Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for lung cancers with KRAS P.G12C mutation. N Engl J Med. 2021;384(25):2371–81.
Strickler JH, Satake H, George TJ, Yaeger R, Hollebecque A, Garrido-Laguna I, et al. Sotorasib in KRAS p.G12C-Mutated Advanced Pancreatic Cancer. N Engl J Med. 2023;388(1):33–43.
Fakih MG, Kopetz S, Kuboki Y, Kim TW, Munster PN, Krauss JC, et al. Sotorasib for previously treated colorectal cancers with KRAS(G12C) mutation (CodeBreaK100): a prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 2022;23(1):115–24.
de Langen AJ, Johnson ML, Mazieres J, Dingemans AC, Mountzios G, Pless M, et al. Sotorasib versus Docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: a randomised, open-label, phase 3 trial. Lancet. 2023;401(10378):733–46.
Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou SI, Pacheco JM, et al. Adagrasib in Non-small-cell Lung Cancer harboring a KRAS(G12C) mutation. N Engl J Med. 2022;387(2):120–31.
Li Z, Song Z, Zhao Y, Wang P, Jiang L, Gong Y, et al. D-1553 (Garsorasib), a potent and selective inhibitor of KRAS(G12C) in patients with NSCLC: phase 1 study results. J Thorac Oncol. 2023;18(7):940–51.
Ou SI, Jänne PA, Leal TA, Rybkin II, Sabari JK, Barve MA, et al. First-in-human phase I/IB dose-finding study of Adagrasib (MRTX849) in patients with Advanced KRAS(G12C) solid tumors (KRYSTAL-1). J Clin Oncol. 2022;40(23):2530–8.
Sacher A, LoRusso P, Patel MR, Miller WH Jr., Garralda E, Forster MD, et al. Single-Agent Divarasib (GDC-6036) in solid tumors with a KRAS G12C mutation. N Engl J Med. 2023;389(8):710–21.
Yaeger R, Weiss J, Pelster MS, Spira AI, Barve M, Ou SI, et al. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N Engl J Med. 2023;388(1):44–54.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–6.
Safi SA, Haeberle L, Goering W, Keitel V, Fluegen G, Stoecklein N et al. Genetic alterations predict long-term survival in Ductal Adenocarcinoma of the pancreatic head. Cancers (Basel). 2022;14(3).
Sato T, Osumi H, Shinozaki E, Ooki A, Shimozaki K, Kamiimabeppu D, et al. Clinical impact of primary Tumor Location and RAS, BRAF V600E, and PIK3CA mutations on epidermal growth factor receptor inhibitor efficacy as third-line chemotherapy for metastatic colorectal Cancer. Anticancer Res. 2021;41(8):3905–15.
Díez-Alonso M, Mendoza-Moreno F, Jiménez-Álvarez L, Nuñez O, Blazquez-Martín A, Sanchez-Gollarte A, et al. Prognostic factors of survival in stage IV colorectal cancer with synchronous liver metastasis: negative effect of the KRAS mutation. Mol Clin Oncol. 2021;14(5):93.
Chen QA, Lin WH, Zhou XX, Cao Z, Feng XL, Gao YB, et al. Outcomes following KRAS(G12C) inhibitor treatment in patients with KRAS(G12C)-mutated solid tumors: a systematic review and meta-analysis. Pharmacol Res. 2024;200:107060.
Zhang SS, Lee A, Nagasaka M. CodeBreak 200: Sotorasib has not broken the KRAS(G12C) Enigma Code. Lung Cancer (Auckl). 2023;14:27–30.
Fakih MG, Salvatore L, Esaki T, Modest DP, Lopez-Bravo DP, Taieb J, et al. Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C. N Engl J Med. 2023;389(23):2125–39.
Sorich MJ, Wiese MD, Rowland A, Kichenadasse G, McKinnon RA, Karapetis CS. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol. 2015;26(1):13–21.
Allegra CJ, Rumble RB, Hamilton SR, Mangu PB, Roach N, Hantel A, et al. Extended RAS Gene Mutation Testing in Metastatic Colorectal Carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update 2015. J Clin Oncol. 2016;34(2):179–85.
Awad MM, Liu S, Rybkin II, Arbour KC, Dilly J, Zhu VW, et al. Acquired Resistance to KRAS(G12C) inhibition in Cancer. N Engl J Med. 2021;384(25):2382–93.
Acknowledgements
Not applicable.
Funding
No specific funding was disclosed.
Author information
Authors and Affiliations
Contributions
Shoutao Dang: Conceptualization (equal); data curation (lead); formal analysis (lead); methodology (equal); software (lead); writing – original draft (lead); writing – review and editing (supporting). Shuyang Zhang: Conceptualization (equal); data curation (supporting); formal analysis (supporting); methodology (equal); software (supporting). Jingyang Zhao: Conceptualization (equal); methodology (equal). Wei Li: Conceptualization (equal); data curation (supporting); formal analysis (supporting); methodology (equal); software (supporting); writing – original draft (supporting); writing – review and editing (lead).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dang, S., Zhang, S., Zhao, J. et al. Efficacy and toxicity of KRASG12C inhibitors in advanced solid tumors: a meta-analysis. World J Surg Onc 22, 182 (2024). https://doi.org/10.1186/s12957-024-03449-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-024-03449-8